Expression, Purification, Analytics
Analytics-driven purification and quality control of your antigen and complex biologics at different research scales with low endotoxin levels

Have you run your biologics discovery program, passed the engineering phase and are now at a stage where you would like to further characterize your biologics?
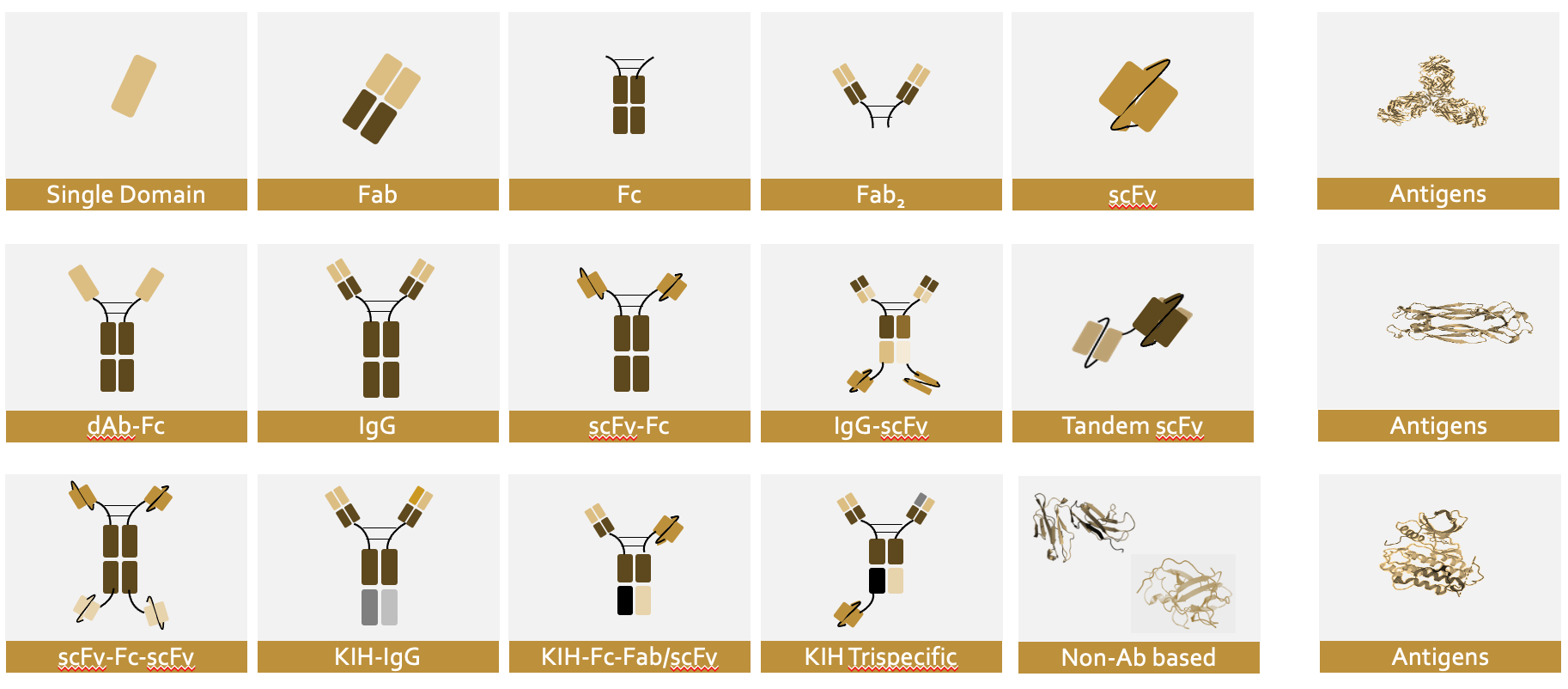
At Proteros we are familiar with a wide variety of biologics formats (bs-Ab, ms-Abs, cytokines, non-human antibody formats and antigens) and deliver the right biologics with accurate assembly, purity, structural and biophysical characterization and early developabilty assessment (non GxP) in a "one-stop-shop" approach to help you REACH RIGHT FASTER for your downstream applications.
Proteros can express and purify your antigen and biologics at different research scales with low endotoxin levels and study the biologics-antigen biophysical/biochemical interaction, conduct structural analysis on epitope binding and stoichiometry, run antibody thermostability and stress tests and conduct early developability assessments for advanced molecules.
Analytics-driven purification and quality control of your antigen and complex biologics at different research scales with low endotoxin levels
Focus on challenging biologics and complex strategies – one-stop-shop for assembly-right biologics products




Cutting-edge analytical platform for "assembly-right" product delivery. Monomer content: >98% via SE-HPLC, Endotoxin-level: down to 0.1 EU/mg. Concentration: UV/Vis @280nm. Purity >98% via CE-SDS (LabChip)

.jpg)

biologics delivered per year
biologics projects per year
meaningful protein, protein-ligand and antigen-antibody structures per year
World Renowned Protein Science Capabilities
Industrialized HEK & CHO Expression Platform
Cutting-Edge Purification And Bioanalytical Capabilities
A global market leader in Structural Biology with premium X-Ray Crystallography and Cryo-EM capabilities & Biophysics platform
Access to Early Biologics Research Developability Assessment (non GxP)